Description
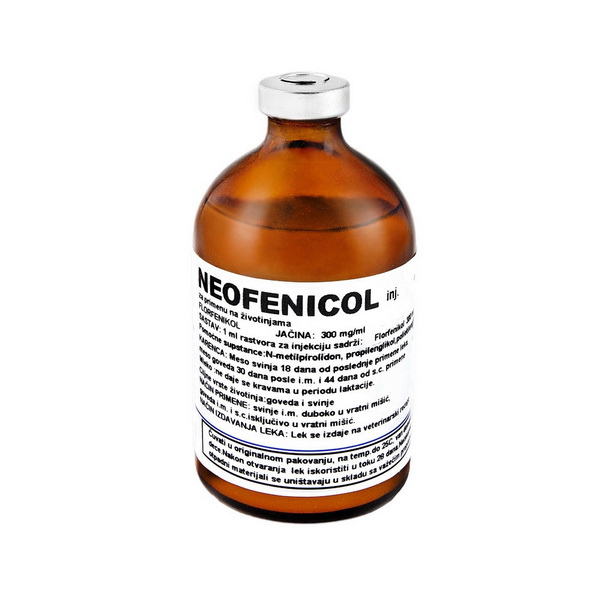
Neofenicol inj.
Composition: 1 ml solution for injection contains:
Florfenicol 300 mg
Indications:
Swine: Treatment of infections caused by strains of Actinobacillus pleuropneumoniae,
Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, Mycoplasma
hyopneumoniae, Mycoplasma hyorhinis, Sallmonella cholerasuis and Streptococcus suis
sensitive to florfenicol.
Cattle: Treatment of bovine respiratory disease (BRD) associated with Pasteurella
haemolytica, Pasteurella multocida and Histophilus Somni (Haemophilus Somnus), the
treatment of bovine interdigital phlegmon (pododermatitis infectious, acute interdigital
nekrobaciloza) provoked by Fusobacterium necrophorum and Bacteroides
melaninogenicus.
Dosage:
Swine: 15 mg florfenicol / kg body weight (1 mL drug / 20kg body weight) twice at an
interval of 48 hours. The total amount of drug administered in one injection site should
not exceed 3 ml.
Cattle: for treatment of bovine respiratory disease ( BRD) and bovine interdigital
phlegmon (foot rot):
Intramuscular applications: 20 mg florfenicol per 1kg body weight (1 mL drug/15 kg bw),
a second dose should be given after 48 hours.
Subcutaneous application: florfenicol 40mg / kg (2 mL of injection /15 kg body weight)
once. The total amount of drug per injection site should not be more than 10mL.
Method of application:
Swine: intramuscular (im) injection, deep into the neck muscle
Cattle: intramuscular (im) or subcutaneously (sc) injection.The injection should be given
only in the neck muscle.
Contraindications: Do not give the boars intended for breeding, as well as piglets that
weight less than 2kg of bw. The drug is not given to breeding cattle and cows during
lactation.
Side effects:
Swine: The most commonly observed side effects are transient diarrhea and / or
perianal and rectal erythema / edema in about 50% of the treated animals, which takes
about a one week .On injection there site may occur swelling that runs for about 5
days.Inflammatory lesions in the application of the drug to pass about 21 days.
Cattle: Reduced food intake and transient softening faeces may occur during therapy.
Treated animals will rapidly and completely recover after completion of therapy.
Intramuscular injection causes swelling at the injection site, which can persist for up to
14 days.Inflammation on injection site may persist for up to 28 days after
use.Subcutaneously use of products can cause swelling and inflammation at the
injection site, which can persist for at least 41 days.
Withdrawal period:
Swine: meat and edible organs are not for human consumption during treatment and 18
days after the last application of the medicine.
Cattle: meat of treated animals not used for human consumption during treatment and
30 days after the last intramuscular injection and 44 days after the last subcutaneous
injection.
Milk: does not give the cows during lactation.
Special warnings
For use in animals:
The product does not interfere with other medications.
The drug does not apply to tiamfenicol and bactericidal drugs (aminoglycoside
antibiotics, beta-lactam antibiotics, fluoroquinolones, polymyxin, etc.). Florfenicol
increases and prolongs action fenition, tolbutamide, chlorpropamide and anticoagulants.
The use of drugs during pregnancy and lactation is not recommended.
Special considerations for people who give animals a drug:
The drug contains substances that irritate the skin and eyes. Avoid direct contact of the
drug with the skin, eyes and clothing. In case of accidental eye exposure, flush with
running water for at least 15 minutes.Skin should be washed with soap and water, and
remove contaminated clothing.In case of persisting irritation,consult a doctor.People
sensitive to propylene glycol and polyethylene glycol should handle preparations with
caution.
Special considerations for storing drugs: Store in original container at temperatures
up to 25 ° C, out of reach of children.
Special precautions for disposal and destruction of drug
Unused drug or drug residues shall be destroyed in accordance with applicable
regulations.
Shelf life: 2 years.
Shelf life after opening: 28 days
Method of issuance: on prescription of veterinarian.
Packaging: bottle of dark glass 20 mL (box 10×20 mL), 50 mL and 100 ml.
ATCvet code: QJ01BA90
Number and date of registration:
10 x 20ml 323-01-0201-11-001 from 10.11.2011.
1 x 50 mL 323-01-0200-11-001 from 10.11.2011.
1 x 100ml 323-01-0199-11-001 from 10.11 2011.
Producer: “FM Pharm”, Subotica, 024/548-130

Reviews
There are no reviews yet.