Description
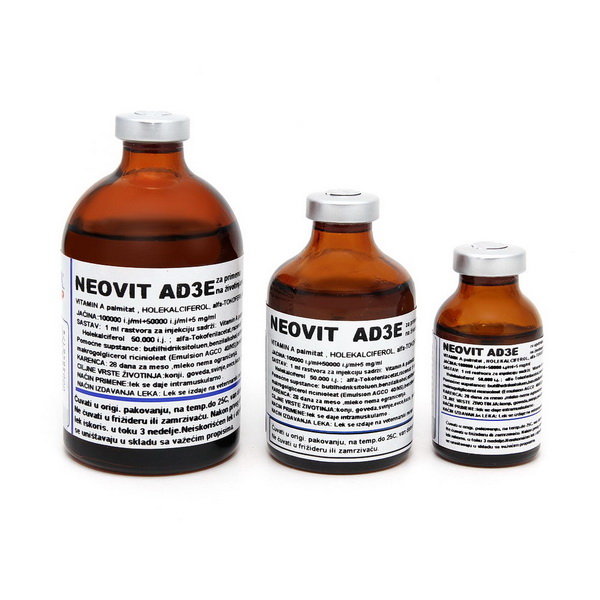
Neovit AD3E
Composition: 1 ml solution for injection contains:
Retinol palmitate (vitamin A) 100,000 i.u
Cholecalciferol (vitamin D3) 50,000 i.u
Alpha-tokoferolacetat, racemate (Vitamin E) 5mg
Indications: Prevention and treatment of hypovitaminosis, rachitis, osteomalacia,
muscular dystrophy and liver dystrophy, keratitis, chicken blindness, breeding diseases,
disorder in fertility and pregnancy. It is used during recuperation period and stress, to
improve the growth of young animals, as well as supportive therapy in various infectious
and parasitic diseases.
Contraindications: Not known
Side effects: During the application of the drug, especially with larger doses at one
injection site, sometimes it can cause local reactions. Anaphylactic reactions are
possible. Very rarely, and only after prolonged administration of high doses, they can
cause side effects, which result from A and D hypervitaminosis (anorexia, nausea,
vomiting, enteritis, diarrhea, cachexia, swelling and bleeding of the gums, arrhythmias,
bone fractures)
Target species: horses, cattle, sheep, goats, pigs, dogs and cats.
Dosage and method of administration:
The medicine is administered i.m. once, in a daily dose of:
Cattle: 3-5 ml per 100 kg of body weight
Horses: 2-4 ml per 100 kg of body weight
Calves and foals: 3-4ml per 50 kg of bw
Sheep and goats: 3-4 ml per 25 kg of bw
Lambs, goats: 1-2 ml per animal
Pigs: 5 ml per 50 kg of bw
Dogs and cats: 0.5 to 2.5 ml kg of bw
In therapy the drug is applied once at the recommended doses, and if necessary the
application can be repeated. Interval between applications should be at least a week.
For preventive purposes medicine is given once, and if necessary, an application can be
repeated every two to three months.
Instructions for proper use of drugs:
When giving a large volume of products, it should be applied on more injection sites.
Withdrawal period: For meat its 28 days. Milk can be used for human consumption
without restrictions.
Special warnings
For use on animals
After prolonged use of high doses there may be signs of hypervitaminosis A and / or
vitamin D.
Retinol reduces anti-inflammatory effect of glucocorticoids.Barbiturates shortens the
biological half-time of cholecalciferol. Nevertheless, tocopherol is a distinct synergist with
selenium.
In the absence of incompatibility studies, the drug must not be mixed with other
veterinary medicinal products.
Use during pregnancy and lactation
Given the fact that tests on laboratory animals indicate teratogenic effects of vitamin A,
use of this drug in pregnancy is recommended only in cases where the vitamin deficit is
clinically confirmed.
Special considerations for persons administering the veterinary medicinal product
to animals
Do not drink, do not eat or smoke when handling the preparation.
After use, wash hands with soap and water.
Special precautions for disposal and destruction of drug
Unused drug or drug residues shall be destroyed in accordance with applicable
regulations.
Special considerations for storing drugs: Store in original container at temperatures
up to 25˚ C, not kept in the refrigerator or freezer.
Keep out of the reach of children.
Shelf life in original packaging: 2 years.
Shelf life after opening: 21 days
Method of issuance: On prescription of veterinarian
Packaging: Glass bottle of dark glass type II, 20ml, 50 ml and 100 ml.
ATCvet code: QA11JA **
Number and date of registration:
1x 100ml 323-01-0595-11-001 from 28.06.2012.
1x 50 ml 323-01-0594-11-002 from 28.06.2012.
1x 20ml 323-01-0593-11-001 from 28.06.2012.
Producer: “FM Pharm”, Subotica, 024/548-130
Reviews
There are no reviews yet.