Description
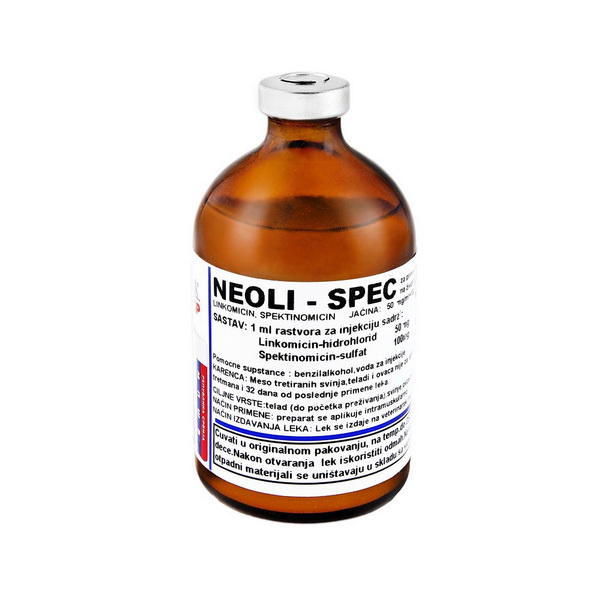
Neoli-spec
Composition: 1 ml solution for injection contains:
Lincomycin hydrochloride 50mg
Spectinomicin sulphate 100mg
Indications: Treatment of primary and secondary infections of pigs, calves, sheep, dogs
and cats, caused by pathogens which are sensitive to the active principles in the
preparation: streptococcus, staphylococcus, E. coli, salmonella, mycoplasma,
Brachyspira hyodisenteriae, etc.
Contraindications: The drug is not administered to animals hypersensitive to the active
principles in the preparation. Hypersensitivity is manifested by pruritus of anus, urticaria,
generalized pruritus and vaginitis.Immediately discontinue application of the drug and
initiate symptomatic therapy. Due to the toxicity of the drug is not given to horses and
small herbivores (rabbits, guinea pigs, hamsters), and patients with renal and hepatic
demage.Strictly is contraindicated in cattle that chew the cud because of ruminants
lincomycin may lead to persistent diarrhea to fatal outcome.
Side effects: At the application site may appear swelling.Sometimes in pigs, especially
after prolonged use, can cause phenomenon soft stools or severe diarrhea. Rarely may
appear irritation (redness) of the skin and swelling of the anus, behavior slightly excited.
These reactions to the drug effect ceased spontaneously within 5-8 days. Lincomycin in
ruminants can lead to persistent diarrhea to fatal outcome.
Target species:
Calves(up to early rumination), pigs, sheep, dogs and cats.
Dosage and method of administration:
The drug is administered i.m. in the following doses:
Pigs: 1ml/10kg of body weight.The dose is repeated every 24 h, treatment lasts 3-5
days.
Calves: 1mL/10kg of body weight, with the first day repeat dose after 12 hours, and then
the dose is repeated every 24 hours.Therapy takes 2-4 days.
Sheep: 1mL/10kg of body weight. The dose is repeated every 24 h, and therapy lasts for
3 days.
Dogs and cats: 1mL/5kg of body weight.The dose is repeated at intervals of 12 to
24h.Therapy takes 3-7 days, and in exceptional cases up to 10 days.
Instructions for proper use of drugs:
At one injection site should not be given more than 20 mL of calves and 10 mL of sheep
and pigs.Larger volume should be divided into several injectable places.The drug is not
given to horses, cattle that began to chew, rabbits, guinea pigs and hamsters.
Withdrawal period: Meat of cattle and sheep is not for human consumption during
treatment or for 21 days after the last treatment, and meat of pigs 14 days.The drug is
not indicated for the sheep whose milk is used for human consumption.
Special considerations:
For use in animals:
In all species after rapid i.v. giving lincomycin may cause cardiovascular disorders.Also,
at all target species may occur diarrhea.The drug should be used only for the target
species for which it is prescribed.
Ccyclamates reduce gastrointestinal absorption of lincomycin .Lincomycin is the
antagonist with erythromycin.Erythromycin may alleviate the inhibitory effects of
lincomycin on protein synthesis in bacteria, probably because it reacts with the same
location in ribosomes.Do not give the drug with macrolide and aminoglycoside
antibiotics.
Use in pregnancy and lactation:
Since lincomycin passes the placental barrier and amniotic fluid can be reached 2/3 of
the concentration in the mother’s blood, is not recommended for use in pregnancy and
lactation. The drug is not given to sheep whose milk is used for human consumption.
Special considerations for people who give animals a drug:
In people who handle the drug may cause anaphylactic reactions and photosensitisation.
Avoid direct contact with skin, mucous membranes and eyes.
Special precautions for disposal and destruction of drug
Unused drug or drug residues shall be destroyed in accordance with applicable
regulations.
Special considerations for storing drugs: Store in original container at temperatures
up to 25 ° C, keep out of reach of children.
Shelf life: 2 years.
Shelf life after first opening: 28 days at temperatures up to 25 ˚ C
Method of issue: on prescription of veterinarian.
Packaging: bottle of dark glass type II of 100 mL and 50 mL, closed with bromobutyl
rubber stopper and aluminum cap.
ATCvet code: QJ01RA94
Number and date of registration:
1x 100ml 323-01-0590-11-001 date 03.08.2012.
1x 50 mL 323-01-0589-11-001 date 03.08.2012.
Producer: “FM Pharm”, Subotica, 024/548-130


Reviews
There are no reviews yet.